Covid-19 vaccine: Ready, set, roll-out
With two vaccines having received emergency use authorisation, India is prepared to meet the logistical challenge. For now, the deployment has begun with the vaccination of healthcare workers.
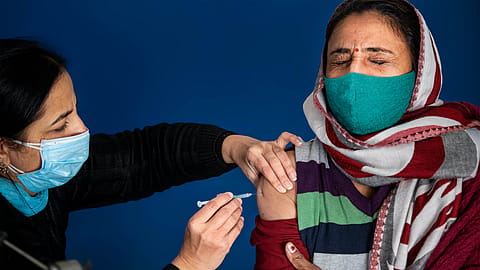
From the moment Chinese scientists sequenced the genome of the SARS-CoV-2 virus, or Covid-19, a year ago, the goal for healthcare teams across governments, the science community, and pharmaceutical companies had been defined: finding a vaccine to control the pandemic.
It has been a long year but that wait seems to be over.
As of January 2021, six vaccines had received emergency use authorisation (EUA) internationally: Russia’s Sputnik V, China’s Sinovac Biotech and Sinopharm, Pfizer-BioNTech, Moderna, and Oxford-AstraZeneca in different markets. In India, the two vaccines with EUAs were Covaxin, developed indigenously by Bharat Biotech India Ltd (BBIL), and Covishield, co-developed by Oxford University and AstraZeneca, and manufactured and marketed in India by Adar Poonawalla’s Serum Institute of India.
Now, the logistics, speed, and efficiency of vaccinations for frontline workers have taken centre stage. State governments, under the direction of the National Expert Group on Vaccination Administration of Covid-19 (NEGVAC), began the vaccination drive on January 16. At this point, India is only at the beginning of the initial phase of vaccination in which 30 million healthcare workers, from the public and private sectors, will be receiving their first shot. In the first six days of the roll-out, over one million had been vaccinated.
A hierarchy of health centres—primary health centres (PHC), subcentres (smaller facilities with lesser infrastructure which operate as subsidiaries of a PHC), and community health centres—has been set up for smooth deployment. (Government-owned facilities are primary sites, while others—a panchayat bhavan, a school or even a private hospital used for vaccination—are called outreach centres.) Union health secretary Rajesh Bhushan has directed state governments to record daily feedback on progress in deployment.
For a sense of Ground Zero, picture the new normal at the Wazirabad vaccination site in Gurugram, Haryana, set up in the primary section of the Wazirabad Government Senior Secondary School. Here, beneficiaries line up in the cordoned-off school compound on Mondays, Thursdays, and Saturdays; these are designated vaccination days so as not to disrupt the regular immunisation initiatives under India’s Universal Immunisation Programme (UIP).
Operations begin at 9 a.m. at the site, and the last dose is administered by 5 p.m. There are checks at every stage, starting with the vaccination officer who ensures that only those names on the list (100 per day) are allowed to move to the second point. There, too, the beneficiary’s identity is verified against any of the 12 photo identification cards specified. An entry is made on the CoWIN app (the vaccine registration platform) downloaded on the healthcare staff’s phones.
The beneficiary then moves to the vaccinator who explains the possible adverse effects after the first dose, and is asked to report any unusual physical discomfort. The beneficiary is also urged to continue following Covid-19 protocols of wearing a mask, social distancing, and maintaining hand hygiene. It is only then that the first dose of the vaccine is injected. The vaccinator enters the date and time into the CoWIN app, and the beneficiary is asked to sit in the observation area for 30 minutes before leaving. The date for the second dose—Day 28 after the first one—is intimated to the beneficiaries.
The flow of movement is in one direction so that no one crosses each other. The site has an ambulance to get anyone to the nearest hospital in case of severe Adverse Effect Following Immunisation (AEFI). There are police personnel for crowd control and each health worker on duty has been sensitised about dealing with unregistered people in a peaceful manner.
Ten days before Fortune India had spent time at the Wazirabad site, the WHO (World Health Organization) country head for India, Dr Roderico H. Ofrin, had expressed satisfaction at India’s preparedness. The WHO team of 1,000 healthcare staff, 280 of whom are medical officers, have been spread across the country, offering support to the government’s efforts. “India has a robust UIP and it has helped in working on the smooth roll-out of the Covid-19 vaccine,” Ofrin tells Fortune India.
Meanwhile, the excitement and satisfaction among the frontline medical workers executing the vaccination drive is palpable. At the Wazirabad site which is in the PHC category, Dr Ashima Sheoran and her team of four doctors, 40 Accredited Social Health Activist (ASHA) workers, 13 Auxiliary Nurse Midwives (ANMs) and a pharmacist have received training about the CoWIN app in addition to their existing knowledge of eVIN (electronic Vaccine Intelligence Network) which the government had adopted almost five years ago.
(INR CR)
Suman Saini, the pharmacist in charge of managing the cold chain, stock, and maintenance of machinery, says that they are used to handling 33 vaccination sessions a month in addition to special national campaigns. “India just concluded 320 million vaccinations for the MMR (measles, mumps and rubella) campaign recently,” says Ofrin. This work has been ongoing even through the pandemic.
Virender Yadav, chief medical officer in Gurugram, believes that successful outcomes of such drives are contingent on preparedness. After all, planning is not limited to paper: the implementation process has to be checked and made foolproof to prevent and handle any untoward incidents. For instance, he says, “Gurugram added a simple protocol, that of keeping two anaphylactic kits, one in the observation area and another where the vaccine is given. The logic being that an AEFI will take place immediately after the dose is injected, if at all.” This practice was added as an all-India recommendation by Ofrin.
Storage of the vaccines—given that they have to be kept at between 2°C and 8°C—is critical. At the Wazirabad site, for instance, an ice-lined refrigerator box contains the vaccines. The bigger picture is that all of India’s over 28,000 cold chain storage points (where a large amount of vaccines can be stored) and nearly 90,000 cold chain units (which cover everything from a freezer to an ice box) have to be pulled in to meet requirements.
These two together include pharmaceutical cold chains, public and private sector hospitals, and medical colleges.
The government allocates vaccines to regional vaccine stores, which are in charge of distributing them to the district stores, and then to the centres. eVIN, which has been used to track vaccine movement, storage, and dispensing for the last three years, has been smoothly “merged into CoWIN, which is the beneficiary interface”, says Dr N.K. Arora, executive director, INCLEN Trust, who has been monitoring the UIP roll-out since the mid-’70s in India.
“The software will be geo-tracking the vaccine,” adds Amit Dinda, professor at the department of pathology, All India Institute of Medical Sciences. That is an essential part of inventory management that will be reflected in the CoWIN dashboard.
As of now, the vaccine regimen is of two doses: the first on Day One and the second on Day 28. “Immunogenicity appears after 14 days of the second dose, on the 42nd day,” says Yadav. Also, there is no interchangeability of multiple vaccine types. And although at this point, there are only two vaccines, Covishield and Covaxin, the plan is to deploy one vaccine per geography to avoid confusion.
The vaccine is not an open vial, which means it cannot be stored beyond a day for use. Waste multiplication factor is estimated at 1.11, allowing 10% of the vaccine to be wasted, according to NEGVAC. This is standard practice in mass vaccination programmes. Specific guidelines have been made for disposal of the vaccine bio-waste safely.
Though it has come with unprecedented challenges, the pandemic has given the pharmaceutical sector the tailwind it desperately needed. The Indian government has announced the setting up of bulk drug manufacturing parks to encourage the production of 41 essential APIs and intermediaries to become self-sufficient. The head of the healthcare practice at Bain & Company, India, Satyam Mehra, points out that “global pharma companies, as well as domestic companies, are looking at alternate sourcing options [to China] for both APIs and intermediates”.
Vaccine manufacturers are already gaining ground. In December, Wockhardt was contracted for two manufacturing lines for the Oxford-AstraZeneca vaccine by the U.K. government. BBIL, even as it ships to India, has signed an agreement with Precisa Medicamentos, a Brazilian company, for the supply of Covaxin with an agreement to prioritise selling through direct procurement by the government of Brazil. And in December, BBIL and Ocugen, a U.S.-based biotech company, signed an agreement to co-develop Covaxin for the American market. Ocugen will have U.S. rights to the vaccine candidate. Dr Reddy’s, with Russia’s Sputnik V, is also applying for EUA in India in the next few weeks.
“The National Digital Health Mission and the setting up of the health stack will drive the creation of multiple business opportunities in the next few years. It can potentially also be a platform for pharmaceutical companies, if the government allows, to engage with patients and doctors to raise awareness and to upskill, respectively,” adds Mehra.
All signs that the disruption started by Covid-19, as far as the Indian healthcare system is concerned, will be visible in the next few years. For now, though, the vaccine remains top of mind.
(This story appeared in Fortune India's February 2021 issue).