QP Pharma cough syrup 'duplicated' to defame India: MD
The cough syrup contained unacceptable amounts of diethylene glycol and ethylene glycol as contaminants, as per WHO.
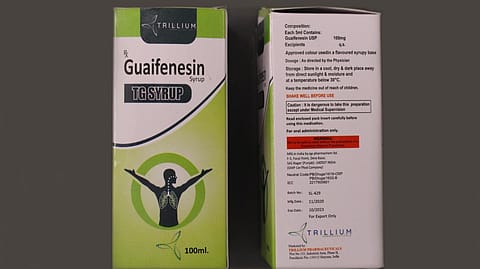
A day after the World Health Organization (WHO) issued its medical product alert for a batch of 'substandard' cough syrup made by QP Pharmachem in Punjab, the company's managing director alleged that someone 'duplicated' the cough syrup to 'defame India'.
"Food And Drug Administration of Punjab doubt that someone has duplicated the product (cough syrup) sent to Cambodia and then sold it in the Marshall Islands and Micronesia to defame the Government of India," Sudhir Pathak, MD of the drugmaker, told news agency ANI.
The cough syrup, manufactured in Punjab's Dera Bassi, is marketed by Haryana-based Trillium Pharma.
According to the company's MD, the drug regulator of Punjab has taken samples of the cough syrup sent to Cambodia for testing. "A total of 18,336 bottles of cough syrup were sent," he told the newswire.
Earlier on Tuesday, the WHO said that samples of the Guaifenesin syrup from the Marshall Islands were analysed by quality control laboratories of the Therapeutic Goods Administration (TGA) of Australia. The analysis found that the product contained unacceptable amounts of diethylene glycol and ethylene glycol as contaminants.
Recommended Stories
Diethylene glycol and ethylene glycol are toxic to humans when consumed and can prove fatal, according to WHO. The 'substandard product' is unsafe and its use, especially in children, may result in serious injury or death, the public health body warned.
Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state and acute kidney injury which may lead to death, it said.
(INR CR)
To date, neither the stated manufacturer nor the marketer has provided guarantees to WHO on the safety and quality of these products, the WHO said, adding that the cough syrup may have marketing authorisations in other countries in the Western Pacific region.
It may have also been distributed, through informal markets, to other countries or regions, the UN's public health agency said.
"If you have the affected product, WHO recommends that you do not use it. If you, or someone you know, has, or may have used the affected product, or suffered an adverse reaction or unexpected side-effect after use, you are advised to seek immediate medical advice from a healthcare professional," the public health body cautioned.
WHO also urged increased surveillance within the supply chains of countries and regions likely to be affected by these products. "Increased surveillance of the informal/unregulated market is also advised. National regulatory authorities/health authorities are advised to immediately notify WHO if these substandard products are discovered in their respective country," it said.